About our group
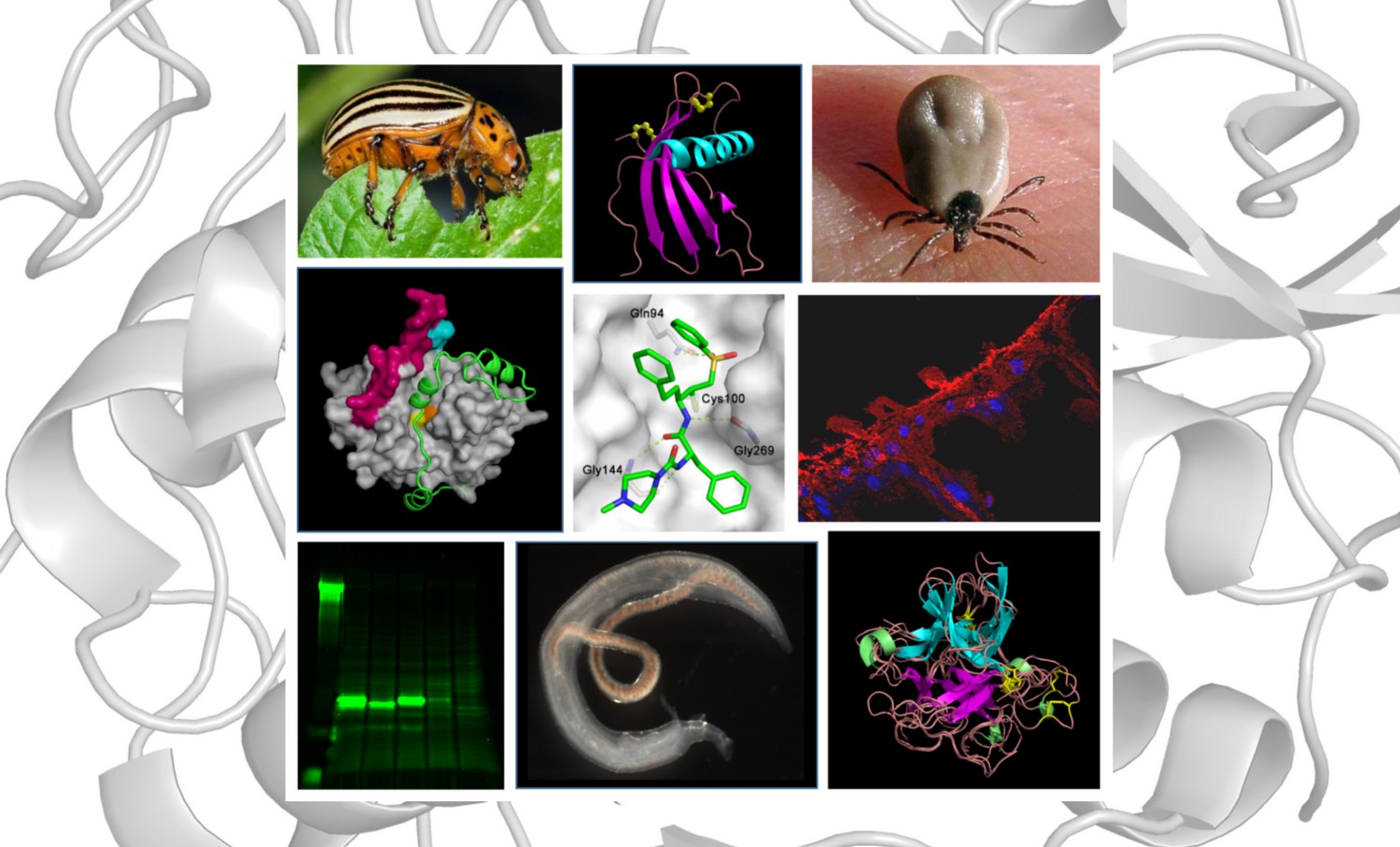
Our research is focused on cathepsin proteases and proteolytic systems controlled by these enzymes. They play a critical role in a number of pathologies such as cancer, osteoporosis, neurodegenerative and immune diseases. Cathepsin-like proteases are also essential for viability or virulence of important human pathogens, parasites and pests. In our work, we employ methods of biochemistry, molecular biology and structural biology to reveal mechanisms of function and natural regulation of cathepsins and related proteases. Armed with this knowledge, we develop cathepsin inhibitors as a molecular tool for new therapeutic strategies.
Publications
All publications
Novel Structural Mechanism of Allosteric Regulation of Aspartic Peptidases via an Evolutionarily Conserved Exosite
Cell Chemical Biology 25 (3): 318-329 (2018)
Pepsin-family aspartic peptidases are biosynthesized as inactive zymogens in which the propeptide blocks the active site until its proteolytic removal upon enzyme activation. Here, we describe a novel dual regulatory function for the propeptide using a set of crystal structures of the parasite cathepsin D IrCD1. In the IrCD1 zymogen, intramolecular autoinhibition by the intact propeptide is mediated by an evolutionarily conserved exosite on the enzyme core. After activation, the mature enzyme employs the same exosite to rebind a small fragment derived from the cleaved propeptide. This fragment functions as an effective natural inhibitor of mature IrCD1 that operates in a pH-dependent manner through a unique allosteric inhibition mechanism. The study uncovers the propeptide-binding exosite as a target for the regulation of pepsin-family aspartic peptidases and defines the structural requirements for exosite inhibition.
Structural Basis for Inhibition of Cathepsin B Drug Target from the Human Blood Fluke, Schistosoma mansoni
Journal of Biological Chemistry 286 (41): 35770-35881 (2011)
Activation Route of the Schistosoma mansoni Cathepsin B1 Drug Target: Structural Map with a Glycosaminoglycan Switch
Structure 22 (12): 1786-1798 (2014)